Abstract
Introduction Chronic graft-versus-host disease (cGVHD) remains a significant limitation to the long-term success of allogeneic hematopoietic cell transplantation (HCT). The incidence and risk factors for cGVHD occurring after a previous diagnosis of steroid-sensitive (SS), steroid dependent (SD), or resistant (SR) acute GVHD (aGVHD) compared to de-novo cGVHD is unknown. We analyzed the impact of aGVHD treatment response on the severity and clinical outcomes of cGVHD occurring after HCT.
Methods The study population included 784 consecutive adult and pediatric first allogeneic HCT recipients with malignant disorders treated at the University of Minnesota between 2008 and 2016. All graft and donor sources, and GVHD prophylaxis strategies after either myeloablative (MAC) or reduced intensity conditioning (RIC) regimens were included. Only aGVHD cases treated with systemic steroids as first-line therapy were included. Our prior study (PMID: 33656537) defined distinct aGVHD treatment response groups based on response to first-line corticosteroids as SS, SR, and SD aGVHD, and we defined the diagnosis and overall severity of cGVHD using the 2014 NIH Consensus Criteria.
Survival was estimated by the Kaplan-Meier method. Cumulative incidence of cGVHD was estimated by cumulative incidence function, with non-chronic GVHD and non-relapse mortality (NRM) as competing risks. Moderate/severe cGVHD free, relapse-free survival (msCRFS) was defined as survival without relapse, death, or moderate/severe cGVHD requiring systemic therapy. Cumulative incidence of cGVHD and msCRFS were analyzed in a landmark analysis starting at 120 days after HCT, by which most aGVHD treatment responses have been ascertained. Fine and Gray proportional hazards regression was used to create a multivariable model for risk of cGVHD. Failure-free-survival (FFS) following chronic GVHD was defined as survival without relapse, death, or new systemic treatment initiated more than 30 days after cGVHD onset. Outcomes following cGVHD were analyzed from the date of cGVHD onset.
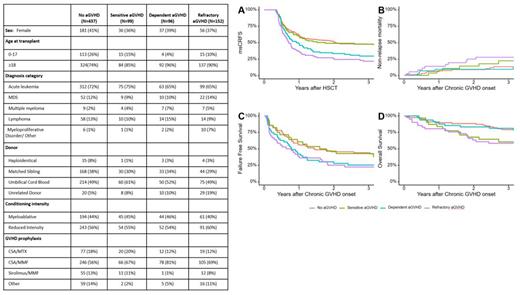
Results Patient and transplant characteristics are shown in Table 1. Among 784 patients, 437 did not develop aGVHD, while 99, 96 and 152 developed SS, SD, or SR aGVHD, respectively. 198 patients developed cGVHD. The 3-year cumulative incidence of any cGVHD was highest in the SD group, followed by SR, SS, and no aGVHD groups (47%, 41%, 32%, 21%, respectively; P < .01). Of the 4 groups, cumulative incidence of both mild and moderate cGVHD was highest among the SD group, while severe cGVHD was highest among the SR aGVHD group. In multivariable analysis, SD aGVHD was independently associated with the highest risk of cGVHD (HR 2.1 vs. no aGVHD; 95% CI, 1.5-3.2; P < .01), followed by SR (HR 1.8 vs. no aGVHD; 95% CI, 1.2-2.7; P < .01), and SS (HR 1.3 vs. no aGVHD; 95% CI, 0.8-2.1; p=0.21). Older age was independently associated with higher risk of cGVHD, while reduced intensity conditioning, umbilical cord blood and matched unrelated donors were associated with lower risk of cGVHD.
3-year CRFS was higher in the no aGVHD and SS groups (each 47%), compared to SD and SR groups (26% and 20%, respectively; p<0.01). Similarly, 3-year msCRFS was higher in the no aGVHD and SS groups (each 48%), compared to SD and SR groups (29% and 22%, respectively; p<0.01). 3-year NRM following cGVHD was highest in the SR group (p=0.05). 3-year FFS was higher in the no aGVHD (44%) and SS groups (43%), compared to SD and SR groups (25% and 22%, respectively; p<0.02). Overall survival after cGVHD was lowest in those with SR and SS aGVHD.
Conclusions We identified SD aGVHD as an independent significant risk factor for development of cGVHD. Among those with cGVHD, cumulative incidence of mild or moderate cGVHD is highest among those with preceding SD aGVHD. Severe cGVHD was most frequent among those with previous SR aGVHD. CRFS and msCRFS were similar in those with no aGVHD or prior SS aGVHD, but msCRFS was worst in the SR group and intermediate in the SD group. Our findings suggest that previous aGVHD response states are important predictors of cGVHD incidence, severity and response as well as overall survival. Improved treatment of aGVHD may limit the incidence and morbidity of subsequent cGVHD.
Disclosures
Weisdorf:FATE Therapeutics: Other: Research Support; Incyte: Other: Research support. Holtan:Vitrac Therapeutics: Research Funding; Incyte: Research Funding; Ossium: Consultancy; Generon: Consultancy; CSL Behring: Other: Clinical trial adjudication.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal